Tomorrow the FDA’s “Vaccines and Related Biological Products Advisory Committee” will review data from Pfizer’s vaccine trial to consider emergency approval of their vaccine for use in the US. The presentation has been released. I have included a link. You may find it interesting and shows the detailed rationale and analysis necessary to gain approval for a medication, device, or vaccine. The US has stricter guidelines for approval than many countries, especially since the thalidomide fiasco in the late 50’s and early 60’s.
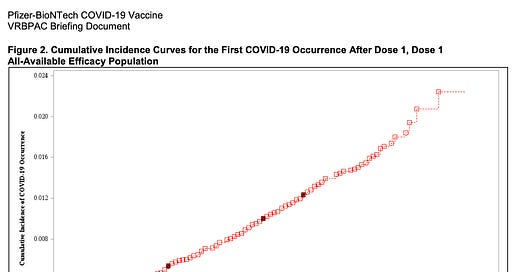
If you look at anything, look at Figure 2 on page 30 that I have copied below. The red line shows the rising number of cases in people who received the placebo and the blue line shows that the number of cases in people who actually received the vaccines starts flattening within 11-14 days after the initial vaccine. The dark spots on each line show the number of serious COVID-19 cases who needed hospitalization- 7 in the placebo group and only 1 in the vaccine arm. Impressive, to say the least. The data also breaks down who was in the study and other data, like side effects such as fever, aches, etc.
Even though other countries are approving vaccines more quickly than us, I agree with the FDA’s thoroughness in the approval process. However, we are in an horrific tailspin and am hopeful that the vaccine can start to reverse our situation. The UK started giving vaccines yesterday and not surprisingly 2 allergic reactions were noted and they have warned people with histories of anaphylaxis to not get the vaccine yet. These less common events will happen as more people get immunized just due to shear numbers increasing the odds of rare events. The company and others will now start to define these reactions better so that they can be better understood and avoided.
I am intrigued and hopeful about the messenger-RNA method of immunization. Even though this may be the first mRNA vaccine approved, it is not the first one tested. Moderna and other companies have been testing mRNA vaccines against more rare diseases, like CMV (cytomegalovirus) which causes disease in transplant patients and other immunocompromised conditions. Also, a new rabies vaccine and one for Zika are being tested and have been promising. These particular COVID-19 vaccines are moving forward more quickly, due to the urgency of the pandemic and the magnitude of infections which allows them to collect data much more quickly. They were closing in on approval for a Zika vaccine, when Zika petered out and sort of disappeared. (If only SARS-CoV-2 virus had done that, but not likely since Zika is passed via mosquito bites and SARS-CoV-2 is via droplets and aerosols).
The mRNA is injected into the muscle of your arm and then enters cells of the immune system and tell that cell to produce a protein that looks like the spike protein of the coronavirus. Next, the spike protein look alike is released from the cell and the body’s immune system then recognizes the spike protein as foreign and makes antibodies against it, neutralizing it. Within the cell that produced the proteins, the mRNA dissipates within 48 hours. Despite rumors, it is not injected to the cells’ DNA. (HIV and other retroviruses do that but in a completely different fashion with specific enzymes that this mRNA will not be able to make.) This initial phase of immune development starts to protect the body, as we have seen, within about 11-14 days. To sustain the immunity, a booster shot is give after 3 weeks.
These are complex processes and may cause an immune response in people-body aches, chills, fevers for a day or two. The new Shingles vaccine does the same thing, although not everyone gets the reaction. Dr. Fauci is hopeful about these vaccines, especially if the FDA approves it after reviewing the data. NPR had an interesting report on the processes that must be done in order to get the vaccine distributed. Dry ice will be an issue because of the low temperature needed to keep Pfizer’s mRNA vaccine stable. It will be thawed before use and can be kept at room temperature for up to 6 hours. Logistically, planning will be needed to avoid wasting precious doses. The government also will only distribute half of the doses at a time to ensure the other half is available for the booster shots 3 weeks later. Such an important detail for those of us who remember the recent shortage of the new Shingles vaccine where people often had to wait a lot longer than the recommended 2-6 months to get their second shot.
And the last link below is a link to a webinar about vaccines from the Fred Hutchinson Research Center and the Washington Department of Health next Tuesday. And this explains the study better than I can.
Wash your hands, cover your nose, keep safe six, stay safe for a while longer.
And finally, my caveat is that this is my experience and my opinions, which are subject to change as more information is available, and not related to the organization I work for. Thanks for reading.

https://www.fda.gov/media/144245/download
https://en.wikipedia.org/wiki/Thalidomide_scandal
https://www.medpagetoday.com/podcasts/trackthevax/90085
https://www.medpagetoday.com/infectiousdisease/covid19/89998
https://www.cbsnews.com/news/covid-vaccine-fauci-what-to-know/
https://www.npr.org/2020/12/02/941716442/before-the-shot-in-the-arm
https://www.reuters.com/article/health-coronavirus-dryice/crimped-u-s-dry-ice-supply-complicates-rural-u-s-vaccine-release-idUSKBN28J1IQ
https://www.nytimes.com/live/2020/12/09/world/covid-19-coronavirus#the-us-will-initially-hold-back-half-the-first-vaccine-supply
https://www.eventbrite.com/e/making-sense-of-vaccines-during-covid-19-tickets-130616267773?utm-medium=discovery&utm-campaign=social&utm-content=attendeeshare&aff=escb&utm-source=cp&utm-term=listing
https://www.businessinsider.com/how-well-pfizer-shot-works-against-covid-19-2020-12